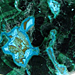
Photo of Chrysocolla in Processed & Rough Form
Photo of Chrysocolla in Processed & Rough Form
Chrysocolla is a mineral with a hardness of 2 out of 10 on the Mohs scale of mineral hardness [?]. These Monoclinicly structured gems are made of hydrated copper silicate, their full chemical compound being (Cu,Al)2H2Si2O5(OH)4.nH2O.
Chrysocolla is a silicate that forms as stalactitic masses, in radiating groups, or closely-packed aggregates. It appears as green, blue, and blue-green, but can also be brown or black when impurities are present. It may be translucent to nearly opaque, and has a vitreous to earthy lustre.
It forms in the oxidation zone of copper deposits, and occurs with azurite, malachite, and cuprite. When decomposed in hycrochloric acid, chrysocolla produces a silica gel.
Large masses of chrysocolla are found in Chuquicamata (Chile) and in the desert mining regions of Arizona, New Mexico, Morocco, and Rhodesia. Deposits are also found in Cornwall (England), Pennsylvania, Utah, and in Italy.
The specific gravity [?] for Chrysocolla is 2.2, its refractive index [?] is 1.57-1.63, and its double refraction [?] is 0.03.
History
It takes its name from the Greek "chrysos" meaning "golden" and "kolla" meaning "glue" from the name of the substance used to fuse gold.
Industrial Usages
Chrysocolla is an important mineral for ore prospectors, as its presence may suggest that porphyry copper ore deposits are close by. But aside from that, it is not considered valuable, though its beautiful shades of blue cause it to be often mistaken for turquoise. While it is too soft to be made into jewelry, chrysocolla found in quartz deposits are often hard enough to polish for cabochons.