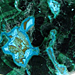
Photo of Phosphophyllite in Processed & Rough Form
Photo of Phosphophyllite in Processed & Rough Form
Phosphophyllite is a mineral with a hardness of 4 out of 10 on the Mohs scale of mineral hardness [?]. These Monoclinicly structured gems are made of hydrated zinc phosphate, their full chemical compound being Zn2(Fe,Mn)(PO4)2.4H2O.
Phosphophyllite is a very rare mineral, a hydrated zinc iron manganese phosphate with a monoclinic crystal system.
It appears as long prismatic or thick tabular crystals. It is colorless to deep bluish-green. Polysynthetic twinned crystals are common.
It is semi-hard, light, with excellent prismatic cleavage. Translucent to transparent with vitreous luster. It turns gray and loses water when heated in a closed tube. Fuses easily and is soluble in most acids.
Phosphophyllite is a secondary mineral derived from the alteration of sphalerite and iron-manganese phosphates.
The finest phosphophyllite crystals in the world, up to 10cm long, transparent and twinned, occur in Potosi, Bolivia. Other localities producing crystallized specimens include Hagendorf, Bavaria (Germany), and North Groton, New Hampshire (USA), Czech Republic, Sweden. Extraordinary crystals have been found in massive sulphide deposits in the Unificada mine, Cerro Rico, Potosi, Morococala mine, Oruro,all in Bolivia, as well as in south Australia, and in Zambia.
The specific gravity [?] for Phosphophyllite is 3.1, its refractive index [?] is 1.59-1.62, and its double refraction [?] is 0.021.
History
Phosphophyllite derives its name from the phosphate in its mineral composition, and the Greek word for "cleavage."
Industrial Usages
Phosphophyllite is sometimes faceted into gems. It is prized by collectors.