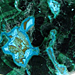
Photo of Pyrite in Processed & Rough Form
Photo of Pyrite in Processed & Rough Form
Pyrite is a mineral with a hardness of 6 out of 10 on the Mohs scale of mineral hardness [?]. These Cubicly structured gems are made of iron sulfide, their full chemical compound being FeS2.
Pyrite is an iron sulphide mineral with a cubic crystal system and is dimorphous with marcasite.
It occurs as cubic crystals with striated faces, or in the form of pentagonal dodecahedra, usually well-crystallized, either isolated or in small, often well-formed groups, or as "iron cross" twins. It is a characteristic, brassy-yellow or pale-gold color, opaque and with a metal luster. It sometimes occurs as nodules or concretions, consisting of aggregates of minute crystals. Occasionally, it has a brown to rust-red weathering skin.
It is quite hard, brittle, and will crumble beneath a hammer blow, unlike gold, with which it has been confused (thus being called "fool's gold"). The powder is (again, unlike gold) black or grayish.
It is paramagnetic, and is insoluble in hydrochloric acid.
In uncut stones, the shape of the crystal combined with the color, luster, and high density, or the form of the nodules, is unmistakable. Even when cut, its appearance is unique.
Pyrite is very common and widely distributed. It is deposited both by magmatic segregation from basic rocks and as a result of intrusive processes in general, especially during hydrothermal phases. It also occurs in sedimentary environments.
It occurs in deposits on its own, in association with other minerals in sulphide ore deposits, in argillaceous and calcareous rocks, in bituminous coal and lignite beds, and as an accessory constituent in many igneous rocks.
Large deposits are found all over the world, but those of Spain, Italy, Norway, Sweden, Germany, Japan, and those of the United States are famous. Very well-formed crystals or nodules are also found away from major deposits. "Thunderbolts" is a local term for small nodules of pyrites found in Sussex, England.
Aside from looking a lot like gold, pyrite is often confused with its dimorph marcasite, a very similar mineeral of the same color which is much less common and has orthorhombic symmetry. The misnomer is now so widespread that it is nearly always referred to by jewelers and traders as marcasite when used for ornamental purposes, even if the obvious cubic crystal form leaves no doubt as to it being pyrite. It is the concreted nodules of sedimentary origin that could occasionally be marcasite. True marcasite is unsuitable for jewelry as it easily powders in air.
The term auriferous pyrite is given to some pyrites if they contain no gold of commercial value.
Pyrite as inclusions are found in quartz, beryl or emerald, sodalite, lapis lazuli, and amber.
How can I tell Pyrite from Gold?
Pyrite has a brassy-yellow color compared to gold's rich yellow, leaves a black streak (gold leaves gold streak), is harder (6-6.5 vs gold's 2.5-3), and forms angular crystals unlike gold's rounded forms. Pyrite also feels lighter and often shows striations on crystal faces.
Why do some Pyrite cubes have striations?
The distinctive striations (parallel lines) on pyrite cube faces are natural growth patterns called striations. These lines run perpendicular on adjacent faces and are a key identifying feature of natural pyrite, rarely seen in other cubic minerals.
Are Pyrite Suns rare?
Pyrite Suns (disc-shaped radiating crystals) are unique to Illinois' coal mines and are highly sought after. True specimens are increasingly rare as mines close. Their unusual flat, radial formation makes them valuable collector's items.
Why does my Pyrite tarnish rainbow colors?
This iridescent tarnish, called peacock ore, occurs when pyrite oxidizes. While beautiful, it indicates the specimen is degrading. Some collectors value this tarnish, though it can eventually lead to specimen deterioration.
Do Spanish Pyrite cubes have special value?
Pyrite from Navajún, Spain, is famous for perfect cubic crystals with mirror-like faces. These specimens command premium prices due to their exceptional formation, size, and aesthetic appeal.
Will Pyrite eventually turn to rust?
Pyrite can decompose in humid conditions, forming iron oxides (rust) and sulfuric acid. Store specimens in dry conditions with silica gel packets. Once degradation begins, it's difficult to stop.
What makes some Pyrite specimens so shiny?
Pyrite's metallic luster comes from its crystal structure. Well-formed faces, especially on cubic crystals, can achieve mirror-like polish naturally. This high reflectivity contributed to its historical use as a decorative material.
The specific gravity [?] for Pyrite is 4.9, its refractive index [?] is None, and its double refraction [?] is None.
History
Pyrite derives its name from the Greek "pyr", meaning "fire", because it is one of the stones that produces sparks when struck by iron.
The Incas used pyrite as mirrors. pyrites or marcasites, often polished,called piedra des Inca, have been found in their tombs.
Industrial Usages
Pyrite is the most important source of sulphur after native sulphur. Because of its frequent association with gold and copper minerals it is locally a gold and copper ore. Weathering of pyrite in sulphide deposits produces, in near-surface zones, the so-called iron hat, limonitic iron ore. Pellets of pressed pyrite dust are treated to produce iron, gold, copper, cobalt, nickel, etc. It is also used as a semiconductor.
It was and still is employed as an ornamental material, on account of its luster, its elegant crystal form, the appearance of some roughly radial concretions or certain groups of crystals, and the fact that it can be cut and polished. It is often cut as small roses or small edging stones, or cabochons of any size, and is prized by collectors.
Sometimes, instead of the pyrite faceted cubes which are normally used, their nodules are used instead. These consist of minute crystals with a flattened radial structure, a minutely granular surface and the characteristic, metallic yellow color. Small stones (2-3mm diameter) have been given a flattened circular, rose cut with only three polished facets on top. They are generally set in metal, for obvious reasons of contrast, and are sometimes merely glued, rather than set, onto their supports. They are often found on necklace clasps and old-fashioned, inexpensive brooches.
Because of its widespread occurrence, pyrite is neither imitated nor produced synthetically.